The training is supported by MTPConnect through the WA Life Sciences Innovation Hub.

This course is for MedTech and HealthTech organisations planning to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Your organisation may be involved in one or more stages of the life-cycle such as design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities (e.g. technical support). ISO 13485 can also be used by suppliers or external parties that provide product, including quality management system-related services to such organisations.
Participants will gain knowledge and learn process steps to enable you to commence your journey to implementing an effective QMS aligned with or certified to ISO 13486:2016.
Applying ISO 13485 helps ensure that customers get consistent, good-quality products and services, which in turn brings many business benefits and commercial success.
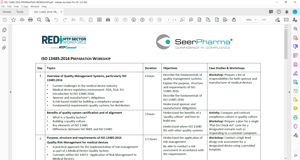
Content
- Overview of Quality Management Systems, particularly ISO 13485:2016
- Benefits of quality system certification and of alignment
- Purpose, structure and requirements of ISO 13485:2016
- Quality Risk Management for medical devices
- Management Responsibility
- Resource Management
- Product realization
- Measurement, Analysis and Improvement
- Overview of application process for ISO 13485:2016
- Development of documentation required for ISO 13485:2016
- Preparation for applying for ISO 13485:2016
Click here for a draft full course outline (PDF)
Participants
This course is open to anyone interested in learning more about the application of ISO 13485:2016 requirements and benefits of a quality management system for medical devices. You may be a subject matter expert, in research, in a start-up, a supplier or a graduate with interest.
Format and Course Length
Delivery will be a mix of theory and interactive case studies and workshops to accelerate your learning with practical skills. The course is a single delivery that will run over 4 consecutive days with group participation exercises and periodic breaks to keep you engaged.
Following successful completion of the 4-day workshop, a digital credential (badge) will be issued from Credly to each participant's registered e-mail address.
What Do I Need?
You just need to attend with a positive attitude and willingness to participate. SeerPharma will provide you with the training materials.
Cost
With support from the WA Life Sciences Innovation Hub, you only need to pay a small co-contribution of $440 (inc. GST) to attend this 4-day workshop. Upfront payment will be required to register and participate. On-site parking at additional cost.
Event Details
Each participant attends all 4 days and sessions will commence at 9am and finish by 5pm each day, local time.
| City | Dates | Venue |
| Perth | 24-27 November 2025 |
Crowne Plaza Perth 54 Terrace Road Perth WA 6000 |
Registration (online credit card payment)
Registration closes Friday 14 November 2025.
Maximum capacity of 25 participants.
You should see a booking window below. If you can't see the booking window, please click here.
Undertake our FREE online Quality Management System (QMS) Primer to understand QMS and ISO 13485 basics first.
Still have questions? Please contact us to discuss your interest and how we can help.